The only glimmer of hope to stop the raging spread of Covid-19 is the rapid development and approval of a vaccine. Three drug companies were able to identify, develop, test and produce vaccines in less than a year which is a paradigm shift in the industry. “To the layperson it may appear drug companies, Clinical Research Organisations (CROs) and the FDA etc. has cut corners to undermine public confidence regarding the safety and efficacy of the vaccines. But nothing is further from the truth,” says the CEO of ClinPro Trials (CPT) www.clinprotrials.com, Monita Dukhia.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210112005099/en/
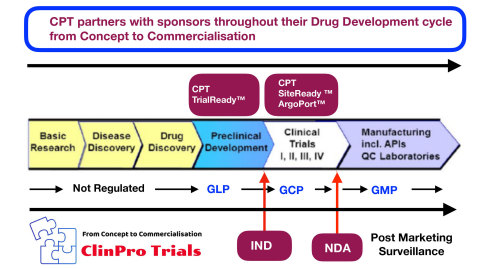
ClinPro Trials partners with Sponsors from Concept to Commercialisation (Graphic: Business Wire)
For a new drug to be developed and brought to market, it undergoes a defined lifecycle from Proof of Concept, to Preclinical animal studies, then clinical trials (Phase 1 to 3). The implementation of proper compliance controls during the study ensures patient rights, safety, and welfare are protected and data integrity and clinical study's compliance with the FDA regulations were followed. If these controls are missing or inadequate, the results of the study cannot be guaranteed and the FDA will reject the new drug. “Compliance controls cannot be built into a study after Phase 3 is over; these controls must be an integral part of it throughout the drug development lifecycle from Concept to Commercialisation,” says the CEO of CPT, Monita Dukhia.“Through preparing for, and hosting FDA BIMO inspections for sponsors for over a decade, we observed the poor compliance and products provided by most CROs during the conduct of clinical trials, resulted in drug approvals being delayed or denied altogether,” she continued.
Due to poor compliance during a clinical study, results are rejected, and phases must be repeated resulting in undue financial burdens and delays for sponsors. The cost of poor compliance is staggering for sponsors. To finance a new drug throughout its lifecycle can cost upwards of 1 billion dollars and take up to 15 years! Given the high stakes of a drug approval being delayed or denied due to poor compliance; it is crucial for sponsors to work with a CRO like CPT whose core competency is compliance. “CPT is spearheading the paradigm shift of implementing the necessary compliance controls into a drug’s development lifecycle from Concept to Commercialisation, so new therapies can move through the drug development cycle in a rapid and compliant manner and pass the FDA BIMO inspection to be approved for market,” says the CEO of CPT, Monita Dukhia. Sponsors should partner with CPT who has the expertise to get it done right the first time thereby reducing costs and timelines. With the Covid-19 vaccines, we witnessed the paradigm shift of a drug’s lifecycle reduced to approximately 10% of its regular time; proving a drug’s lifecycle time can be drastically reduced if the sponsor partners with the right CRO and other stakeholders during their drug development lifecycle.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210112005099/en/
Contacts:
415 350 9536