- Blister pack supports treatment discretion and portability for some people living with HIV
ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced Dovato (dolutegravir/ lamivudine) is now available in a blister pack in the U.S. Dovato is approved as a complete regimen to treat HIV-1 infection in adults with no antiretroviral (ARV) treatment history or to replace the current ARV regimen in those who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable ARV regimen with no history of treatment failure and no known resistance to any component of Dovato.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240124470296/en/
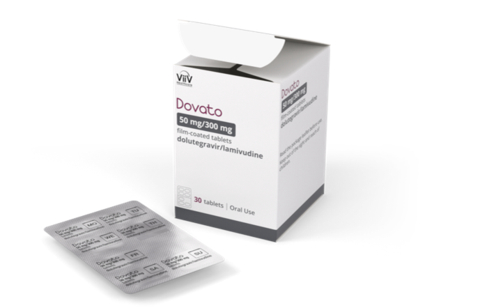
Dovato Blister Pack Packaging and Product (Photo: Business Wire)
Lynn Baxter, Head of North America at ViiV Healthcare, said: “The Dovato blister pack is designed to help address some of the challenges we hear from the HIV community, which include stigma and convenience, and offers a discreet package which may fit more seamlessly into people’s daily routines. Everyone has different experiences and preferences when it comes to their HIV treatment and, at ViiV Healthcare, we are pleased to offer a variety of treatment and packaging options that help suit the needs of people living with HIV.”
The Dovato blister pack is a monthly 30-count box containing five sheets of tablets. Created based on insights from the HIV community, each sheet is about the size of a credit card and perforated, and designed to be small, discreet, and not bulky. The sheets allow people living with HIV to view the number of pills left and track their doses.
The FDA approved the Dovato blister pack on November 3, 2023. ViiV Healthcare plans to offer the Dovato blister pack in some European markets in 2024. ViiV Healthcare will continue to offer Dovato in the 30-count pill bottle.
About Dovato (dolutegravir/lamivudine)
Dovato is a once-daily, single-pill, 2-drug regimen (2DR) that combines the integrase strand transfer inhibitor (INSTI) dolutegravir with the nucleoside reverse transcriptase inhibitor (NRTI) lamivudine.
Dovato is approved in the U.S., Europe, Japan, Australia and other countries worldwide.
Trademarks are owned by or licensed to the ViiV Healthcare group of companies.
Dovato (dolutegravir and lamivudine) tablets
INDICATION
Dovato is indicated as a complete regimen to treat HIV-1 infection in adults with no antiretroviral (ARV) treatment history or to replace the current ARV regimen in those who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable ARV regimen with no history of treatment failure and no known resistance to any component of Dovato.
IMPORTANT SAFETY INFORMATION
BOXED WARNING: PATIENTS CO-INFECTED WITH HEPATITIS B VIRUS (HBV) AND HIV-1: EMERGENCE OF LAMIVUDINE-RESISTANT HBV AND EXACERBATIONS OF HBV
All patients with HIV-1 should be tested for the presence of HBV prior to or when initiating Dovato. Emergence of lamivudine-resistant HBV variants associated with lamivudine-containing antiretroviral regimens has been reported. If Dovato is used in patients co-infected with HIV-1 and HBV, additional treatment should be considered for appropriate treatment of chronic HBV; otherwise, consider an alternative regimen.
Severe acute exacerbations of HBV have been reported in patients who are co-infected with HIV-1 and HBV and have discontinued lamivudine, a component of Dovato. Closely monitor hepatic function in these patients and, if appropriate, initiate anti-HBV treatment.
Contraindications
- Do not use Dovato in patients with previous hypersensitivity reaction to dolutegravir or lamivudine
- Do not use Dovato in patients receiving dofetilide
Warnings and precautions
Hypersensitivity Reactions:
- Hypersensitivity reactions have been reported with dolutegravir and were characterized by rash, constitutional findings, and sometimes organ dysfunction, including liver injury
- Discontinue Dovato immediately if signs or symptoms of severe skin or hypersensitivity reactions develop, as a delay in stopping treatment may result in a life-threatening reaction. Clinical status, including liver aminotransferases, should be monitored and appropriate therapy initiated
Hepatotoxicity:
- Hepatic adverse events have been reported, including cases of hepatic toxicity (elevated serum liver biochemistries, hepatitis, and acute liver failure), in patients receiving a dolutegravir-containing regimen without pre-existing hepatic disease or other identifiable risk factors
- Patients with underlying hepatitis B or C or marked elevations in transaminases prior to treatment may be at increased risk for worsening or development of transaminase elevations with use of Dovato. In some cases, the elevations in transaminases were consistent with immune reconstitution syndrome or hepatitis B reactivation, particularly in the setting where anti-hepatitis therapy was withdrawn
- Monitoring for hepatotoxicity is recommended
Embryo Fetal Toxicity:
- Assess the risks and benefits of Dovato and discuss with the patient to determine if an alternative treatment should be considered at the time of conception through the first trimester of pregnancy due to the risk of neural tube defects
- Pregnancy testing is recommended before initiation of Dovato. Individuals of childbearing potential should be counseled on the consistent use of effective contraception
Lactic Acidosis and Severe Hepatomegaly With Steatosis:
Fatal cases have been reported with the use of nucleoside analogs, including lamivudine. Discontinue Dovato if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity develop, including hepatomegaly and steatosis in the absence of marked transaminase elevations.
Adverse Reactions or Loss of Virologic Response Due to Drug Interactions with concomitant use of Dovato and other drugs may occur (see Contraindications and Drug interactions).
Immune Reconstitution Syndrome, including the occurrence of autoimmune disorders with variable time to onset, has been reported with the use of Dovato.
Adverse reactions
The most common adverse reactions (incidence ≥2%, all grades) with Dovato were headache (3%), nausea (2%), diarrhea (2%), insomnia (2%), fatigue (2%), and anxiety (2%).
Drug interactions
- Consult full Prescribing Information for Dovato for more information on potentially significant drug interactions
- Dovato is a complete regimen. Coadministration with other antiretroviral medications for the treatment of HIV-1 infection is not recommended
- Drugs that induce or inhibit CYP3A or UGT1A1 may affect the plasma concentrations of dolutegravir
- Administer Dovato 2 hours before or 6 hours after taking polyvalent cation-containing antacids or laxatives, sucralfate, oral supplements containing iron or calcium, or buffered medications. Alternatively, Dovato and supplements containing calcium or iron can be taken with food
Use in specific populations
- Pregnancy: There are insufficient human data on the use of Dovato during pregnancy to definitively assess a drug-associated risk for birth defects and miscarriage. An Antiretroviral Pregnancy Registry has been established. Advise individuals of childbearing potential of the potential risk of neural tube defects. Assess the risks and benefits of Dovato and discuss with the patient to determine if an alternative treatment should be considered at the time of conception through the first trimester of pregnancy or if pregnancy is confirmed in the first trimester
- Lactation: Breastfeeding is not recommended due to the potential for HIV-1 transmission, developing viral resistance in HIV-positive infants, and adverse reactions in a breastfed infant
- Females and Males of Reproductive Potential: Pregnancy testing is recommended before initiation of Dovato. Counsel individuals of childbearing potential taking Dovato on the consistent use of effective contraception
- Renal Impairment: Dovato is not recommended for patients with creatinine clearance <30 mL/min. Patients with a sustained creatinine clearance between 30 and 49 mL/min should be monitored for hematologic toxicities, which may require a dosage adjustment of lamivudine as an individual component
- Hepatic Impairment: Dovato is not recommended in patients with severe hepatic impairment (Child-Pugh Score C)
About ViiV Healthcare
ViiV Healthcare is a global specialist HIV company established in November 2009 by GSK (LSE: GSK) and Pfizer (NYSE: PFE) dedicated to delivering advances in treatment and care for people living with HIV and for people who are at risk of acquiring HIV. Shionogi became a ViiV shareholder in October 2012. The company’s aims are to take a deeper and broader interest in HIV and AIDS than any company has done before and take a new approach to deliver effective and innovative medicines for HIV treatment and prevention, as well as support communities affected by HIV.
For more information on the company, its management, portfolio, pipeline, and commitment, please visit viivhealthcare.com.
About GSK
GSK is a global biopharma company with a purpose to unite science, technology, and talent to get ahead of disease together. Find out more at gsk.com.
Cautionary statement regarding forward-looking statements
GSK cautions investors that any forward-looking statements or projections made by GSK, including those made in this announcement, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Such factors include, but are not limited to, those described under Item 3.D “Risk factors” in the company's Annual Report on Form 20-F for 2022, and Q4 Results for 2023.
Registered in England & Wales: |
|
GSK plc |
ViiV Healthcare Limited |
No. 3888792 |
No. 06876960 |
|
|
Registered Office: |
|
GSK plc |
ViiV Healthcare Limited |
980 Great West Road |
GSK Medicines Research Centre |
Brentford, Middlesex |
Gunnels Wood Road, Stevenage |
United Kingdom |
United Kingdom |
TW8 9GS |
SG1 2NY |
View source version on businesswire.com: https://www.businesswire.com/news/home/20240124470296/en/
Contacts
ViiV Healthcare enquiries:
Media enquiries:
Rachel Jaikaran +44 (0) 78 2352 3755 (London)
Audrey Abernathy +1 919 605 4521 (North Carolina)
GSK enquiries:
Media enquiries:
Tim Foley +44 (0) 20 8047 5502 (London)
Simon Moore +44 (0) 20 8047 5502 (London)
Dan Smith +44 (0) 20 8047 5502 (London)
Sarah Clements +44 (0) 20 8047 5502 (London)
Kathleen Quinn +1 202 603 5003 (Washington DC)
Lyndsay Meyer +1 202 302 4595 (Washington DC)
Alison Hunt +1 540 742 3391 (Washington DC)
Investor Relations:
Nick Stone +44 (0) 7717 618834 (London)
James Dodwell +44 (0) 20 8047 2406 (London)
Mick Readey +44 (0) 7990 339653 (London)
Josh Williams +44 (0) 7385 415719 (London)
Camilla Campbell +44 (0) 7803 050238 (London)
Steph Mountifield +44 (0) 7796 707505 (London)
Jeff McLaughlin +1 215 751 7002 (Philadelphia)
Frannie DeFranco +1 215 751 4855 (Philadelphia)



