
Beam Therapeutics Inc. (NASDAQ: BEAM) is a biotech company that specializes in developing precision gene therapies for hereditary diseases. The medical sector company is a pioneer in a proprietary NASDAQ: CRSP">gene-editing technique called base editing. Unlike CRISPR/Cas-9, popularized by CRISPR Therapeutics AG (NASDAQ: CRSP), molecular scissors cut out specific NASDAQ: BEAM">sequences of the human genome, base-editing allows for single-base (letter) changes without causing double-strand breaks and risking unintended consequences of mutations or off-target effects.
Beam is Benefiting From Big Partners
The company is partnered with Eli Lilly & Co. (NYSE: LLY), Verve Therapeutics as well as Pfizer Inc. (NYSE: PFE) and Apellis Pharmaceuticals Inc. (NASDAQ: APLS) on several projects. Beam's recent Q4 2023 earnings report was similar to CRISPR Therapeutics, with positive EPS and multifold revenue growth. However, just like CRISPR Therapeutics, the majority of the revenue was comprised of licensing fees rather than sales. Check out the sector heatmap on MarketBeat.
The Company Reported a Surprising Profit Windfall
On February 27, 2024, Beam Therapeutics reported Q4 2023 EPS of $1.73 versus consensus analyst estimates for a loss of 69 cents. This was a $2.42 EPS beat. Revenues surged $1,481% YoY to $316.2 million versus $34.16 million consensus estimates. Revenues were comprised mostly of Eli Lilly buying rights to a group of gene-editing therapies that were co-developed with Verve Therapeutics. This transaction included $214.4 million in revenue. The rest of the revenues were derived from payments from partners Apellis and Pfizer. This revenue surge cut Beam's 2023 net loss by more than half, down to $132.5 million.
Beam is Building a Cash Runway
The company still has a war chest of $1.2 billion in cash and cash equivalents. This gives the company a cash runway into 2027. This includes milestone funding for its BEAM-101, BEAM-301, BEAM-302 and ESCAPE therapies and continued investments in its manufacturing capabilities and platform advancements.
The Company Gave an Update on Expected 2024 Milestones
Beam provided an overview of anticipated key milestones it hopes to achieve in 2024 by franchise.
For the sickle cell disease (SCD) franchise, Beam expects to complete patient dosing in the sentinel cohort and begin dosing in the expansion cohort patients in 1H 2024 for the BEACON Phase 1/2 clinical trials for BEAM-101. The editing approach seeks to reactivate fetal hemoglobin. Beam will report the initial data on multiple patients for the trial in 2H 2024. CRISPR Therapeutics and bluebird bio (NASDAQ: BLUE) NASDAQ: BLUE">received FDA approvals on December 8, 2023, for their respective SCD gene therapies.
It also hopes to initiate Phase 1-enabling preclinical studies as it continues to invest in its Engineered Stem Cell Antibody Paired Evasion (ESCAPE) conditioning platform.
For the genetic disease franchise, Beam has filed the European clinical trial application for BEAM-302 for the treatment of alpha-1 antitrypsin deficiency (AATD). AATD is a genetic disorder that affects the lungs and liver. AAT is produced in the liver and is essential, helping to protect against lung damage from protease enzymes. AATD patients produce deficient AAT, which impairs their protective function and can lead to chronic obstructive pulmonary disease (COPD) and emphysema.
Upon CTA acceptance, the BEAM-302 Phase 1 clinical trial is expected to commence in 1H 2024. The company also expected to submit an investigational new drug (IND) application for BEAM-301 for the treatment of glycogen storage disease type 1a (GSD1a) in 1H 2024.
What Did Beam's CEO Have to Say?
Beam Therapeutics CEO John Evans commented, "This year has the potential to be transformative for Beam as we work to advance multiple base editing programs in the clinic, anchoring key high-value franchises through near-term catalysts, all backed by a strong balance sheet."
Evan continued, "In 2024, we expect to initiate our first in vivo clinical studies and report the first-in-human data from our ex vivo base editing clinical programs."
JP Morgan Upgraded BEAM Stock
On January 29, 2024, JP Morgan noted that the AATD treatment candidate has the potential to be a $12 billion commercial opportunity as JP Morgan expects an early biomarker readout from its Phase 1 study. Analysts believe that each 10% incremental probability of success adjusted is worth around $14 per share. JP Morgan upgraded shares of BEAM to Overweight with a $40 price target.
Beam Therapeutics analyst ratings and price targets are at MarketBeat. Beam Therapeutics peers and competitor stocks can be found with the MarketBeat stock screener.
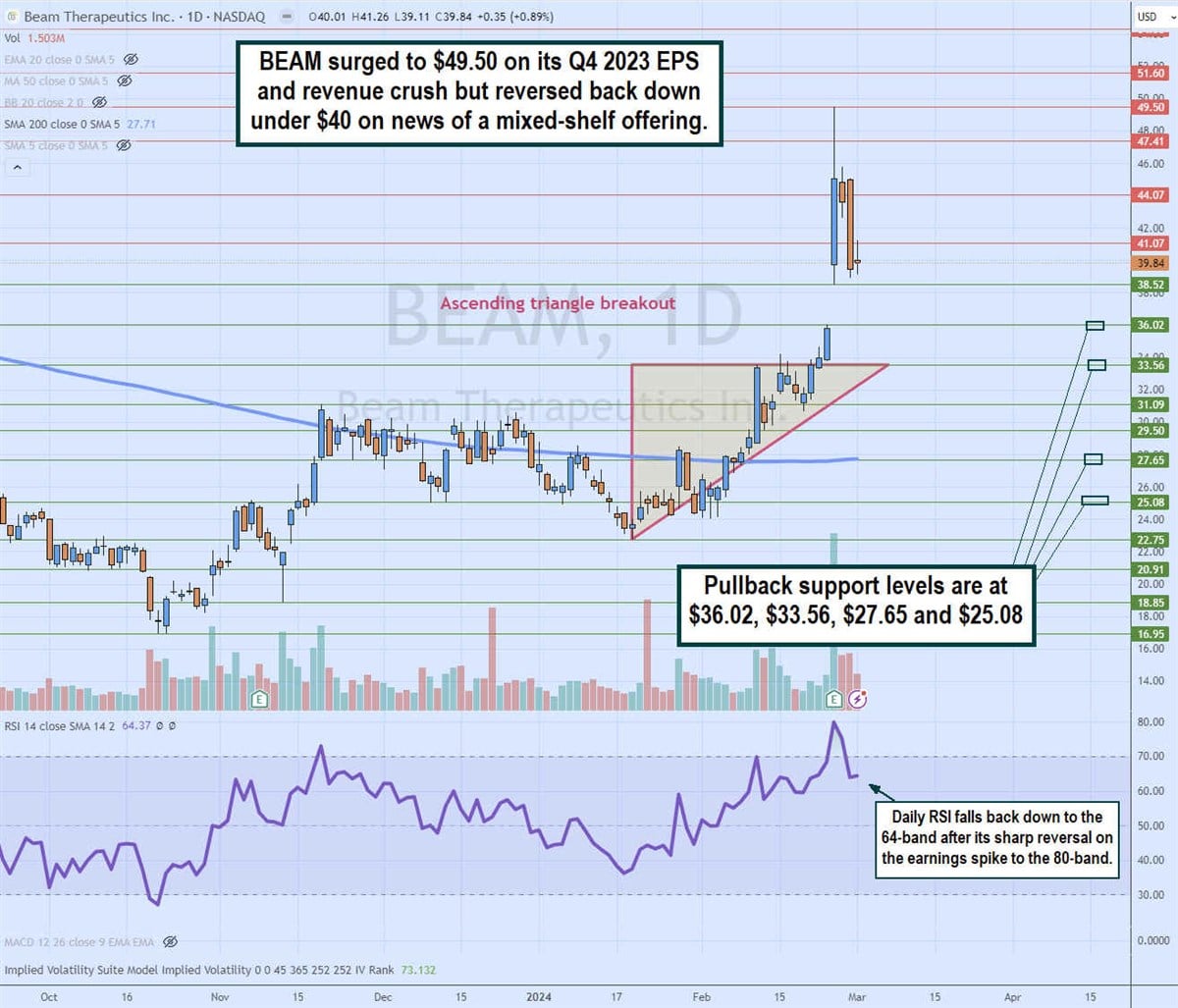 \
\
Daily Ascending Triangle Breakout
The daily candlestick chart for BEAM illustrates a daily ascending triangle breakout pattern. The lower ascending trendline commenced at $22.75 on January 19, 2024, rising through the daily 200-period moving average (MA) at $27.65 towards the flat-top upper trendline resistance at $33.56. The Q4 2023 earnings release caused shares to gap up to $38.52 and surge as high as $49.50. The daily relative strength index (RSI) surged to the 80-band and made a sharp reversal back down to the 64-band as shares reversed back to the $39.84 level on news of its mixed-shelf offering. Pullback support levels are at $36.02, $33.56, $27.65 and $25.08.




