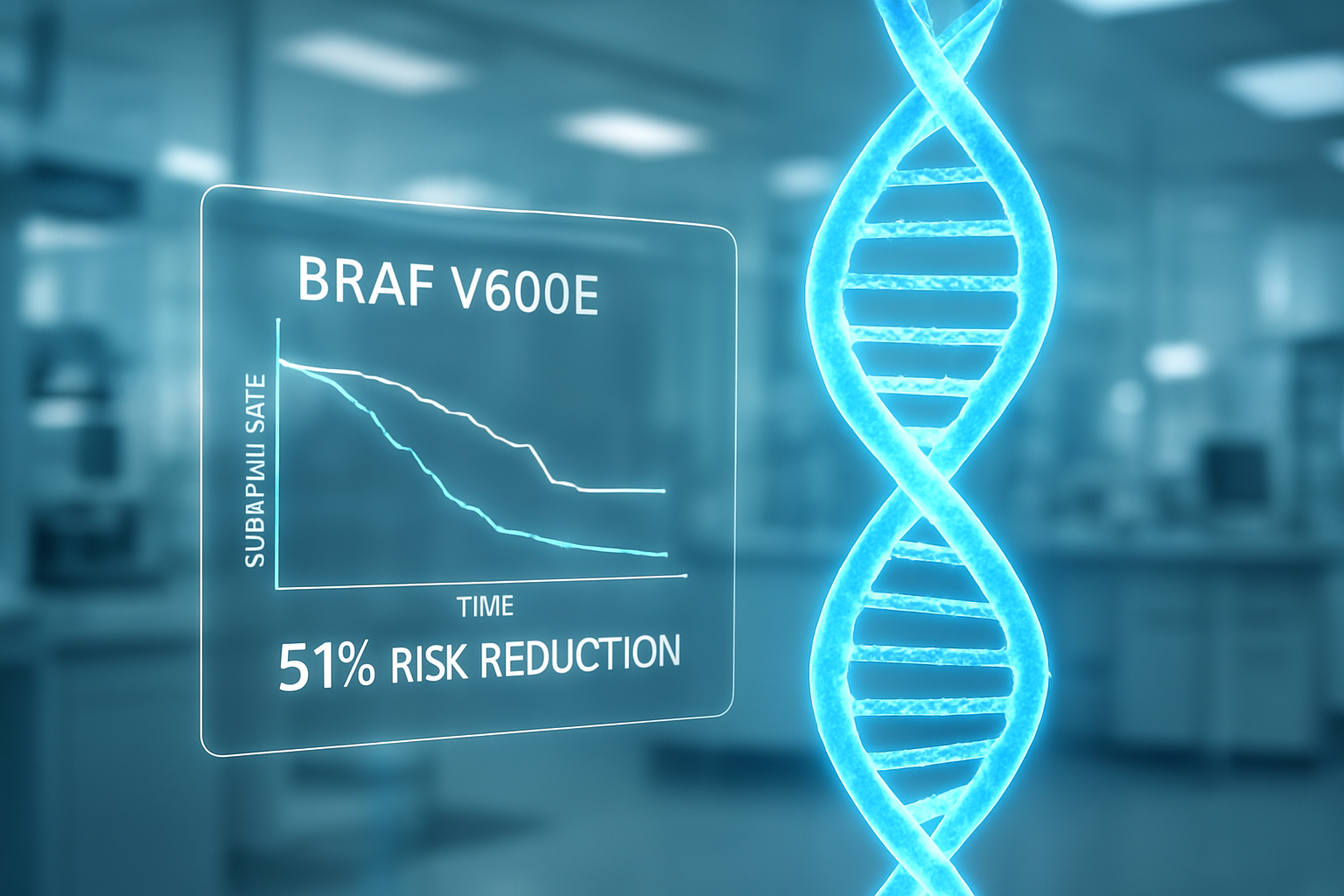
NEW YORK — Pfizer Inc. (NYSE: PFE) has announced breakthrough results from its Phase 3 BREAKWATER clinical trial, demonstrating that its BRAFTOVI (encorafenib) regimen significantly improves both progression-free survival (PFS) and overall survival (OS) in patients with BRAF V600E-mutant metastatic colorectal cancer (mCRC). The data, bolstered by a fresh topline report in February 2026, marks a pivotal shift in the treatment paradigm for one of the most aggressive and difficult-to-treat subsets of colorectal cancer.
The clinical win comes at a critical juncture for the pharmaceutical giant as it aggressively pivots its portfolio toward oncology to offset declining revenues from its COVID-19 franchise. By demonstrating a 51% reduction in the risk of death compared to standard chemotherapy, the BRAFTOVI-based triplet regimen is expected to accelerate regulatory labeling expansions and drive rapid clinical uptake across the United States.
A New Standard of Care: The BREAKWATER Results
The BREAKWATER trial specifically targeted treatment-naïve patients with the BRAF V600E mutation, which affects approximately 10% of the metastatic colorectal cancer population and is historically associated with a poor prognosis. In the primary analysis presented at major medical congresses and updated in early 2026, the combination of BRAFTOVI plus cetuximab and mFOLFOX6 (a standard chemotherapy) achieved a median overall survival of 30.3 months. This is a staggering improvement over the 15.1 months observed in the control group receiving standard-of-care chemotherapy with or without bevacizumab.
The momentum continued into February 2026, with Pfizer reporting positive topline results for "Cohort 3" of the study, which utilized a FOLFIRI chemotherapy backbone instead of mFOLFOX6. This additional data provides oncologists with critical flexibility, allowing for the use of different chemotherapy bases while maintaining the efficacy of the BRAFTOVI/cetuximab targeted core. The hazard ratio for death in the primary triplet arm stood at 0.49, signaling a definitive clinical benefit that analysts believe will make this regimen the undisputed first-line standard for this mutation.
Industry reaction has been overwhelmingly positive. Oncology experts noted that the trial not only met its primary endpoints but did so with a safety profile that was consistent with previous studies. The ability to double the median overall survival in a metastatic setting is a rare feat in solid tumor oncology, positioning Pfizer to dominate this niche market for the remainder of the decade.
Winners and Losers in the Targeted Oncology Race
Pfizer (NYSE: PFE) stands as the primary beneficiary of these results. With the BRAFTOVI/MEKTOVI franchise already generating hundreds of millions in annual revenue, the expansion into first-line mCRC is projected to push the franchise past the $3 billion annual sales mark. This success validates Pfizer’s $43 billion acquisition of Seagen and its broader strategy to deliver eight or more oncology blockbusters by 2030.
Eli Lilly and Company (NYSE: LLY) also stands to gain, as its drug Erbitux (cetuximab) is a required component of the successful BREAKWATER regimen in the United States. While Lilly has faced biosimilar pressure on older products, the formal integration of Erbitux into a first-line "gold standard" for BRAF-mutant patients provides a stable and potentially growing revenue stream within its oncology segment.
Conversely, traditional chemotherapy providers and manufacturers of older anti-VEGF therapies like bevacizumab—marketed as Avastin by Roche (OTC: RHHBY)—may see a contraction in their specific use-case for this mutation. While chemotherapy remains a component of the BRAFTOVI triplet, the move toward highly specific, mutation-driven targeted therapy reduces the reliance on broad-spectrum agents that were previously the only option for these patients.
Precision Medicine and the Strategic Pivot
The success of BRAFTOVI is a cornerstone of the broader "Precision Oncology" movement. For years, colorectal cancer treatment followed a "one-size-fits-all" approach, but the BREAKWATER data underscores the necessity of genomic testing at the point of diagnosis. By isolating the BRAF V600E mutation, Pfizer has turned a high-risk clinical profile into a treatable condition with nearly triple the typical progression-free survival (12.8 months for the triplet vs. 7.1 months for standard chemo).
This event also highlights the intensifying competition in the BRAF-inhibitor space. While Novartis (NYSE: NVS) has seen success with its Tafinlar/Mekinist combination in melanoma and other indications, Pfizer’s focus on the colorectal niche has allowed it to carve out a leadership position where others have struggled. The integration of targeted therapies with existing chemotherapy "backbones" is becoming the blueprint for modern oncology drug development, moving away from monotherapies in favor of synergistic combinations.
Furthermore, these results help insulate Pfizer against the upcoming "patent cliff" for its current blockbusters, such as Ibrance. By building a diverse pipeline that includes ADCs (antibody-drug conjugates) from the Seagen deal and targeted small molecules like BRAFTOVI, Pfizer is successfully rebranding itself as an "Oncology Powerhouse" for the late 2020s.
The Road to Full Regulatory Approval
The immediate next step for Pfizer is the submission of a supplemental New Drug Application (sNDA) to the U.S. Food and Drug Administration (FDA) to convert its existing accelerated approval into full approval and to expand the label to include the FOLFIRI-based regimen. Given the strength of the survival data (OS), many analysts expect a priority review, with a potential label expansion by the second half of 2026.
Physician uptake is expected to be swift. Major clinical guidelines, such as those from the National Comprehensive Cancer Network (NCCN), often update their recommendations shortly after Phase 3 data is published in peer-reviewed journals. If BRAFTOVI is elevated to a "Category 1" recommendation for first-line BRAF-mutant mCRC, it will effectively mandate coverage by most U.S. insurers, removing a significant barrier to commercial growth.
However, challenges remain. The complexity of administering a triplet or quadruplet regimen requires significant coordination in a clinical setting. Pfizer will need to focus its commercial efforts on educating community oncologists—who treat the majority of cancer patients in the U.S.—on the management of side effects and the importance of early genetic screening to identify eligible patients.
Investment Outlook and Summary
The BREAKWATER trial results represent a definitive "win" for Pfizer and a beacon of hope for patients with BRAF-mutant colorectal cancer. The data confirms that targeted therapy, when combined with optimized chemotherapy, can fundamentally change the trajectory of metastatic disease. For investors, this provides much-needed evidence that Pfizer’s massive investment in oncology is beginning to yield high-value, high-margin clinical results.
Key takeaways for the market include:
- Survival Superiority: The 51% reduction in mortality risk sets a high bar for any future competitors in the BRAF V600E space.
- Pipeline Synergy: The success of BRAFTOVI complements Pfizer's growing ADC and bispecific portfolio, creating a comprehensive oncology ecosystem.
- Market Dynamics: Expansion into first-line treatment significantly increases the eligible patient population, supporting the $3 billion+ peak sales forecast.
In the coming months, investors should watch for the formal FDA filing and any additional data regarding the long-term durability of the response. As Pfizer continues its transition away from its pandemic-era identity, clinical milestones like BREAKWATER will be the primary drivers of the stock’s valuation and the company's long-term viability in the competitive healthcare sector.
This content is intended for informational purposes only and is not financial advice.




